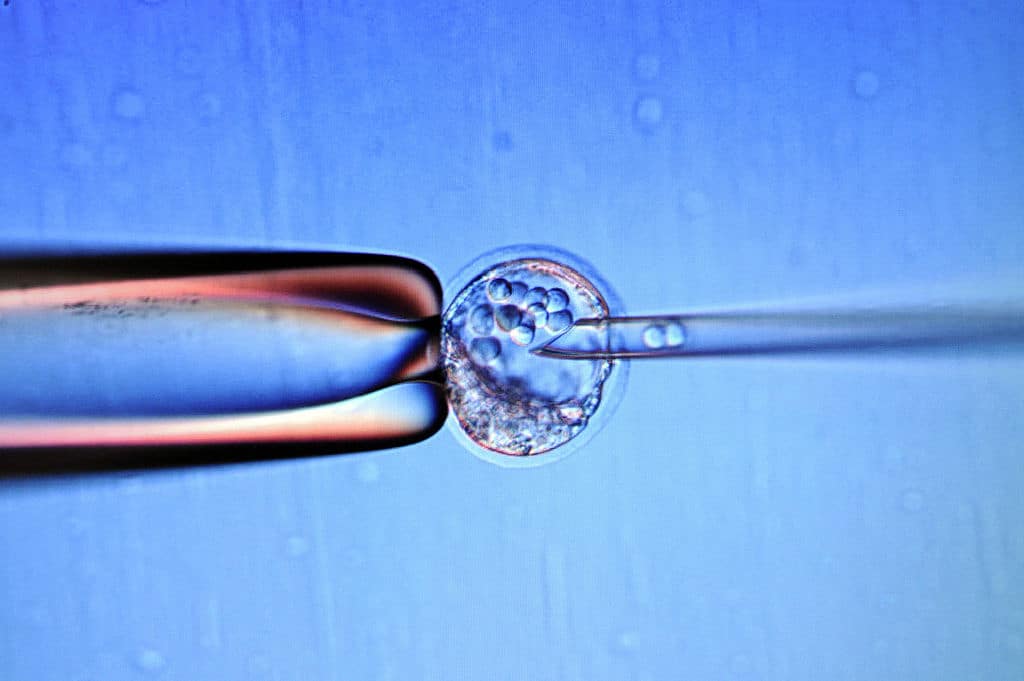
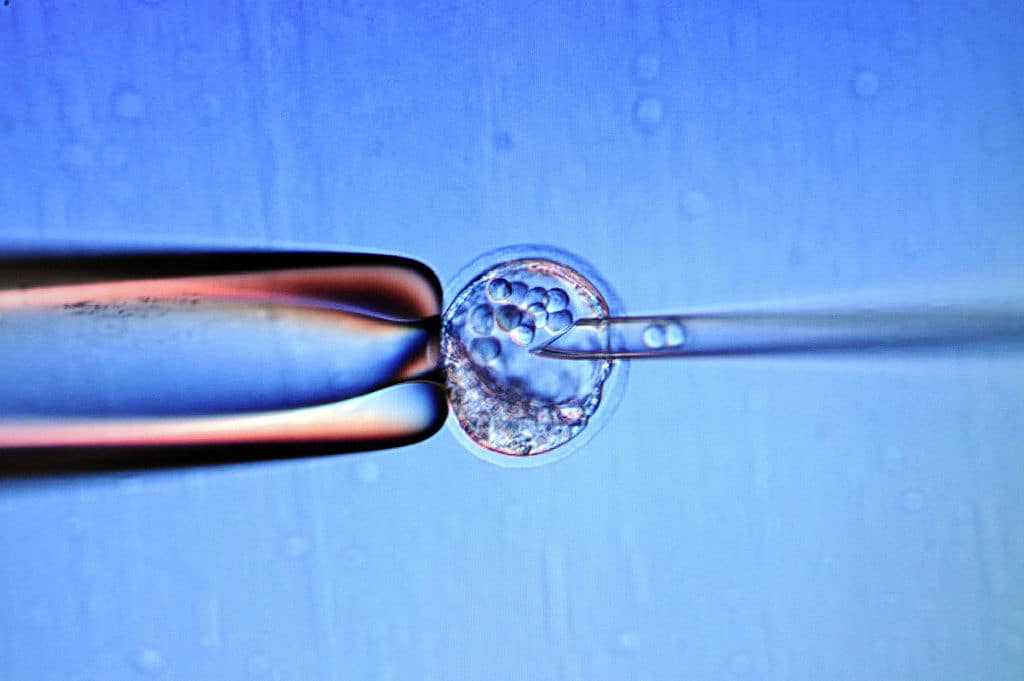
A world-leading Catholic bioethical institute has severely criticised proposals to radically deregulate destructive experimentation on human embryos.
A consultation by the Human Fertilisation and Embryology Authority (HFEA) proposes the abolition of the 14-day upper time limit on experiments on foetuses and human-monkey hybrid embryos conceived outside the womb.
The regulatory quango also wants to permit the creation of genetically-modified babies by legalising germline genome editing for reproduction.
It also seeks to dramatically widen its powers so that it has control over ethical fertility services which do not offer IVF and so it can grant indefinite licences to clinics which do, or which might carry out destructive experiments on human embryos.
The Anscombe Bioethics Centre, an Oxford-based institute serving the Catholic Church in the UK and Ireland, has now criticised the recommendations as “extreme and highly unethical”.
Professor David Albert Jones, the director, said: “The United Kingdom has an ignominious history of support for eugenics and unethical experimentation on human embryos, including generating embryos with human and nonhuman genes.
“Now the HFEA, which is supposed to place ethical restrictions on research, is proposing to allow regulations for such experiments to be permitted without any developmental time limit.
“We urge anyone concerned about these proposals to respond to the HFEA consultation and to express their views in the strongest possible terms.”
The HFEA consultation on the revision of the Human Fertilisation and Embryology Act 1990 ends on Friday April 14.
Anscombe argues that the proposals effectively advocate amending the HFE Act so that it no longer focuses on the special status of the human embryo, the interests of children born through IVF, or the maintenance of public trust.
Deregulation of experiments on human or human-primate hybrid foetuses would, under the plans, go way beyond the first two weeks of life and extend at least up to the point of viability, which in British law is 24 weeks.
Such experimentation would be permitted without either full parliamentary scrutiny, according to Anscombe, or with the informed consent of the biological parents.
The proposed extension of HFEA powers would release the quango from the requirement to make site visits on fertility treatment facilities or experimental laboratories every two years and the authority to licence experimental treatments which have no proven benefit.
Anscombe said in a statement to the press that “to not state the strongest disagreement with these proposals, would be to tacitly accept them”.
“The consultation is framed to discourage, as far as possible, participation from the wider public,” a spokesman said.
“In the interests of democratic debate, and the common good, it is imperative that as many people as possible respond to the HFEA, and that civil society is informed about the extreme and potentially harmful implications of their proposals.
To enable this, the Anscombe Centre has produced a briefing on the consultation which includes its recommendations and advice about how to make a submission.
Julia Chain, the HFEA chair, said however that reform was necessary because “scientific and social attitudes move on far quicker than law” and that “under the current regime, innovation is left in the waiting room”.
She said: “We are consulting on policies that would future-proof UK fertility law, making it agile to scientific development, and able to offer improvements to patient care much more quickly.”
She added: “Regulation would better support innovation and patients, if the law explicitly provided for the HFEA to pilot novel processes for a trial period, with appropriate controls and conditions.
“This is because it can be hard to determine whether a process will achieve the stated aims in practice based just on scientific data.”
(Getty)
A world-leading Catholic bioethical institute has severely criticised proposals to radically deregulate destructive experimentation on human embryos.
A consultation by the Human Fertilisation and Embryology Authority (HFEA) proposes the abolition of the 14-day upper time limit on experiments on foetuses and human-monkey hybrid embryos conceived outside the womb.
The regulatory quango also wants to permit the creation of genetically-modified babies by legalising germline genome editing for reproduction.
It also seeks to dramatically widen its powers so that it has control over ethical fertility services which do not offer IVF and so it can grant indefinite licences to clinics which do, or which might carry out destructive experiments on human embryos.
The Anscombe Bioethics Centre, an Oxford-based institute serving the Catholic Church in the UK and Ireland, has now criticised the recommendations as “extreme and highly unethical”.
Professor David Albert Jones, the director, said: “The United Kingdom has an ignominious history of support for eugenics and unethical experimentation on human embryos, including generating embryos with human and nonhuman genes.
“Now the HFEA, which is supposed to place ethical restrictions on research, is proposing to allow regulations for such experiments to be permitted without any developmental time limit.
“We urge anyone concerned about these proposals to respond to the HFEA consultation and to express their views in the strongest possible terms.”
The HFEA consultation on the revision of the Human Fertilisation and Embryology Act 1990 ends on Friday April 14.
Anscombe argues that the proposals effectively advocate amending the HFE Act so that it no longer focuses on the special status of the human embryo, the interests of children born through IVF, or the maintenance of public trust.
Deregulation of experiments on human or human-primate hybrid foetuses would, under the plans, go way beyond the first two weeks of life and extend at least up to the point of viability, which in British law is 24 weeks.
Such experimentation would be permitted without either full parliamentary scrutiny, according to Anscombe, or with the informed consent of the biological parents.
The proposed extension of HFEA powers would release the quango from the requirement to make site visits on fertility treatment facilities or experimental laboratories every two years and the authority to licence experimental treatments which have no proven benefit.
Anscombe said in a statement to the press that “to not state the strongest disagreement with these proposals, would be to tacitly accept them”.
“The consultation is framed to discourage, as far as possible, participation from the wider public,” a spokesman said.
“In the interests of democratic debate, and the common good, it is imperative that as many people as possible respond to the HFEA, and that civil society is informed about the extreme and potentially harmful implications of their proposals.
To enable this, the Anscombe Centre has <a href="https://bioethics.org.uk/research/reports-submissions/a-briefing-on-the-human-fertilisation-embryology-authority-hfea-consultation-modernising-the-regulation-of-fertility-treatment-and-research-involving-human-embryos/">produced a briefing on the consultation</a> which includes its recommendations and advice about how to make a submission.
Julia Chain, the HFEA chair, said however that reform was necessary because “scientific and social attitudes move on far quicker than law” and that “under the current regime, innovation is left in the waiting room”.
She said: “We are consulting on policies that would future-proof UK fertility law, making it agile to scientific development, and able to offer improvements to patient care much more quickly.”
She added: “Regulation would better support innovation and patients, if the law explicitly provided for the HFEA to pilot novel processes for a trial period, with appropriate controls and conditions.
“This is because it can be hard to determine whether a process will achieve the stated aims in practice based just on scientific data.”
<em>(Getty)</em>